CONRAD is a boutique hybrid of academia, non-profit, pharmaceutical/biomedical R&D, and sociobehavioral research/human-centered design encompassing end-to-end expertise. Our holistic approach to our work makes our products, technologies, and research solutions more innovative, cutting edge and likely to add real value in underserved and low-resource settings.



What We Do

Translational & preclinical research
Mucosal susceptibility to HIV; novel mucosal safety endpoints; PK & efficacy models; novel adherence biomarkers

Product development & quality assurance
Pharmaceutical & combination drug-device product development; technology transfer, set up, validation, and quality monitoring of cGMP manufacturing of clinical supplies

IND enabling
Testing plan design, implementation & oversight for pharmaceuticals, medical devices & combination drug-device products

Adherence support & acceptability testing
End user research to support product development, uptake, and adherence

Regulatory submissions
Documentation to support IND, IDE, NDA, and other regulatory requirements

Clinical studies
Exploratory, phase I, II, and III clinical trials, and demonstration/implementation studies
Highlights
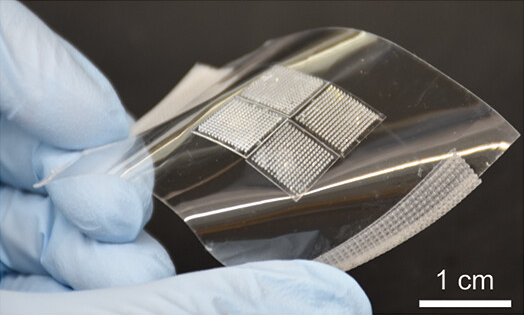 The MAMA Project (Advancing Multi-bNAbs Microneedle Patch Technology For HIV-1 Prevention in Breastfeeding Infants) – NIH 1R01AI186784
The MAMA Project (Advancing Multi-bNAbs Microneedle Patch Technology For HIV-1 Prevention in Breastfeeding Infants) – NIH 1R01AI186784
 First-in-Class Intravaginal Ring containing Tenofovir and Levonorgestrel for prevention of HIV and unintended pregnancy
First-in-Class Intravaginal Ring containing Tenofovir and Levonorgestrel for prevention of HIV and unintended pregnancy

