


Leaders in Global Health and Innovation

Who We Are
CONRAD is a cross-functional R&D team with diverse expertise, brought together by a shared passion for our mission to fill unmet global health needs.

What We Do
Our holistic approach to our work makes our products, technologies, and research solutions more innovative, cutting edge and likely to add real value in underserved and low-resource settings.

Areas of R&D
CONRAD’s products are designed primarily for women in low-resource settings with an emphasis on safety, effectiveness, affordability, and acceptability/user-friendliness.

Our Projects
CONRAD’s R&D programs endeavor to improve global health, in particular women’s and reproductive health, by developing and testing innovative user-centered technologies.

Publications
To contribute to the invaluable fields we work in, CONRAD disseminates its results extensively through high-quality, timely publications in reputable open-access journals.
Highlights
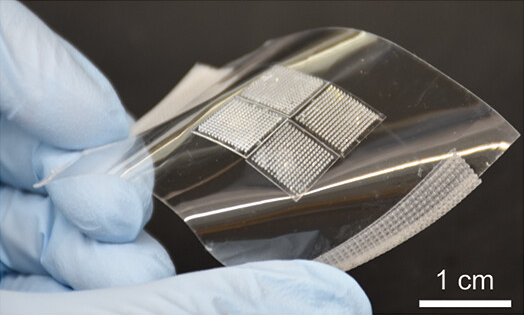 The MAMA Project (Advancing Multi-bNAbs Microneedle Patch Technology For HIV-1 Prevention in Breastfeeding Infants) – NIH 1R01AI186784
The MAMA Project (Advancing Multi-bNAbs Microneedle Patch Technology For HIV-1 Prevention in Breastfeeding Infants) – NIH 1R01AI186784
 First-in-Class Intravaginal Ring containing Tenofovir and Levonorgestrel for prevention of HIV and unintended pregnancy
First-in-Class Intravaginal Ring containing Tenofovir and Levonorgestrel for prevention of HIV and unintended pregnancy
Areas of Impact
Multipurpose Prevention Technologies
CONRAD is currently developing several combination products providing for multiple indications at once, including contraception and protection against HIV and/or other STIs.
Contraception
CONRAD has contributed critical data to approval of many safe/affordable contraceptive devices, such as the PATH Woman’s Condom, Lea’s Shield, Femcap, and SILCS diaphragm (Caya)
Maternal and Neonatal Health
CONRAD works collaboratively with clinical researchers to investigate innovative diagnostic and therapeutic options to improve maternal and neonatal health outcomes.
Women’s Reproductive Health
Our HCD-focused approaches enfranchise women to exercise control over their own sexual and reproductive health and improve their access to safe, affordable health products.
STI Prevention
Our mission to improve reproductive health globally has seen CONRAD researching and developing novel microbicide delivery systems for the prevention of herpes and other STIs.
HIV Prevention
For almost 40 years, CONRAD has served as a leader in the HIV prevention research field, advancing several cutting-edge compounds to late phase trials, such as the tenofovir gel.

Your Partner in Game-Changing Global Health R&D
Thought Leaders
CONRAD has long served as a resource and leader in the fields of reproductive health, especially in women; HIV/STI prevention; contraception; preclinical & clinical product development; and novel drug delivery systems.
Ideas Incubator
Our innovative approaches and product development strategies are based on users' needs, from our proof-of-concept work to R&D, yielding a unique portfolio of drug platforms, APIs, and MPTs.
End-to-End Expertise
Our team manages a variety of R&D phases: from late discovery to preclinical/early clinical testing, evaluating safety, acceptability, and effectiveness, product development, human-centered design, regulatory strategy, and through implementation.
History of Success
As a non-profit R&D organization long serving as a critical resource and leader in the field of reproductive health since 1986, we've built a wealth of experience that contributes to the development of crucial user-centered technologies.

