CONRAD, in collaboration with Dr. Thanh Nguyen from the University of Connecticut and Dr. Francois Villinger from the University of Louisiana at Lafayette, has initiated the development of a cocktail of broadly neutralizing antibodies (bnAbs) delivered by a microarray patch for the prevention of HIV in breastfeeding infants, with the support of an NIH R01 grant. This innovative concept builds upon preliminary data obtained under NIX HIV, a project funded by the US Agency for International Development (USAID), and is a valuable diversification of our ARV-based portfolio. The MAMA Project represents a concerted effort to improve global health, with Dr. Gabriella Scarlatti of the San Raffaele Scientific Institute, Milan providing expert advice on HIV-prevention in infants and the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center (VRC) supplying the necessary bnAbs.
Globally, approximately 130,000 new HIV infections of children occur per year. This exceedingly vulnerable population is much less likely to receive successful treatment than adults, and children account for a tragically disproportionate amount of AIDS-related deaths (13%). A significant number of HIV infections are transmitted by mothers that remain undiagnosed for HIV before, during, or after pregnancy, mothers who acquired HIV during pregnancy or breastfeeding, or mothers who are unable to adhere to antiretroviral therapy. Despite the increasing effectiveness of prevention efforts across the board, neonatal HIV transmission continues to occur during breastfeeding, which accounts for about half of all pediatric HIV infections, and progress has plateaued.
There are many limitations plaguing current pediatric ARV formulations like daily nevirapine and zidovudine, which are shorter acting, require daily intake, are difficult to adhere to, show undesirable side effects, and are associated with stigma in certain areas, which needs to be overcome to better protect vulnerable newborns. Passive immunization with long-acting bnAbs (LS-versions) represents a promising potential strategy for HIV prevention that provides longer lasting protection, high efficacy, and the ability to avoid the adverse effects of ARVs, and overall could be easier to adhere to. This innovative method of postnatal HIV-1 prophylaxis is already being explored in clinical trials such as PedMAb1 (PACTR2022057152787), a phase I safety and pharmacokinetics study investigating administration of one or two bnAbs to newborns through repeated single-dose subcu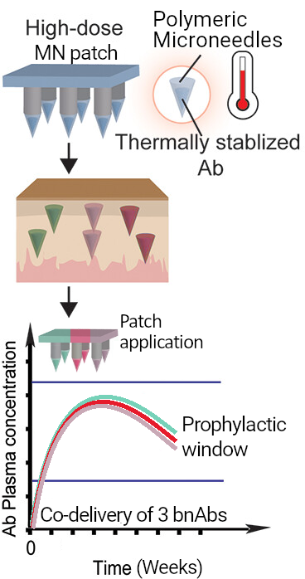 taneous injections in South Africa.
taneous injections in South Africa.
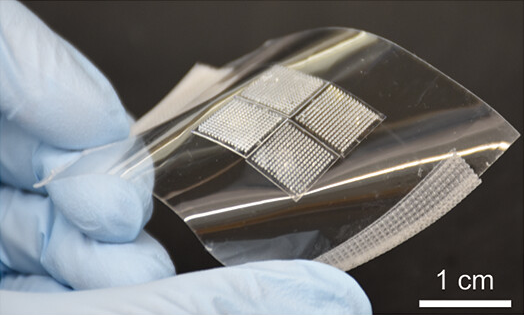
CONRAD’s game-changing innovation comes in the form of a stable, user-friendly, easy-to-administer system for the effective delivery of multiple anti-HIV-1 bnAbs. A novel transdermal microneedle patch able to deliver three thermally stabilized long-acting bnAbs will provide an alternative to daily ARVs that does not require subcutaneous injections with hypodermic needles that cause pain and distress to infants, repeated visits to medical facilities, or highly trained personnel for administration, all of which represent a significant burden on patients and the health-care system, leading to low uptake, adherence, and continuation problems.
Despite many favorable characteristics, there have been relatively few successes in employing microneedle patches, but CONRAD’s previous work has shown that the limitations holding back the filling of this important gap in prophylactic options can be overcome by the right technical innovations (Tran et al., Mol Pharm 2023). Ultimately, the MAMA Project, supported by the expertise of CONRAD investigators and strong external collaborators, will provide proof-of-concept for an effective anti-HIV passive immunization strategy for infants at risk for vertical transmission through breastfeeding, achievable by co-administration of three long-acting bnAbs through a minimally invasive transdermal microneedle patch platform.